Our Clinical Studies
Patients taking ACCRUFeR® (ferric maltol) saw significant improvements in their hemoglobin and iron levels during multiple studies.
ACCRUFeR achieved FDA approval based on three key studies in patients with iron deficiency (ID) and iron deficiency anemia (IDA). All patients in the 3 studies had both ID and IDA.

ACCRUFeR: An effective low-dose treatment for ID and IDA
Could ACCRUFeR be right for you?
ACCRUFeR was studied in patients with iron deficiency or iron deficiency anemia and an underlying condition – either inflammatory bowel disease (IBD) or chronic kidney disease (CKD). However, it’s also approved for other adults with ID or IDA.
Patients with these underlying conditions often have trouble addressing ID or IDA, but in these studies, ACCRUFeR helped them increase their hemoglobin and iron levels.
Studies of patients with iron deficiency and IBD
After 12 weeks
- ACCRUFeR helped patients rapidly increase their hemoglobin levels, achieving meaningful results as early as Week 4. By Week 12, 66% of patients* reached normal hemoglobin levels.
- ACCRUFeR also increased other iron measures, including iron stores (ferritin) and available iron (TSAT).
Please note that ACCRUFeR should not be taken during an active IBD flare.
ACCRUFeR increased hemoglobin** by
compared with a 0.06 g/dL increase for patients on a placebo.
of patients achieved normal hemoglobin levels
compared with 13% of patients on a placebo.
*Out of 64 patients
**Average change at Week 12
Hemoglobin Levels Over Time*
By the end of the 64-week study
of patients were maintaining normal hemoglobin levels
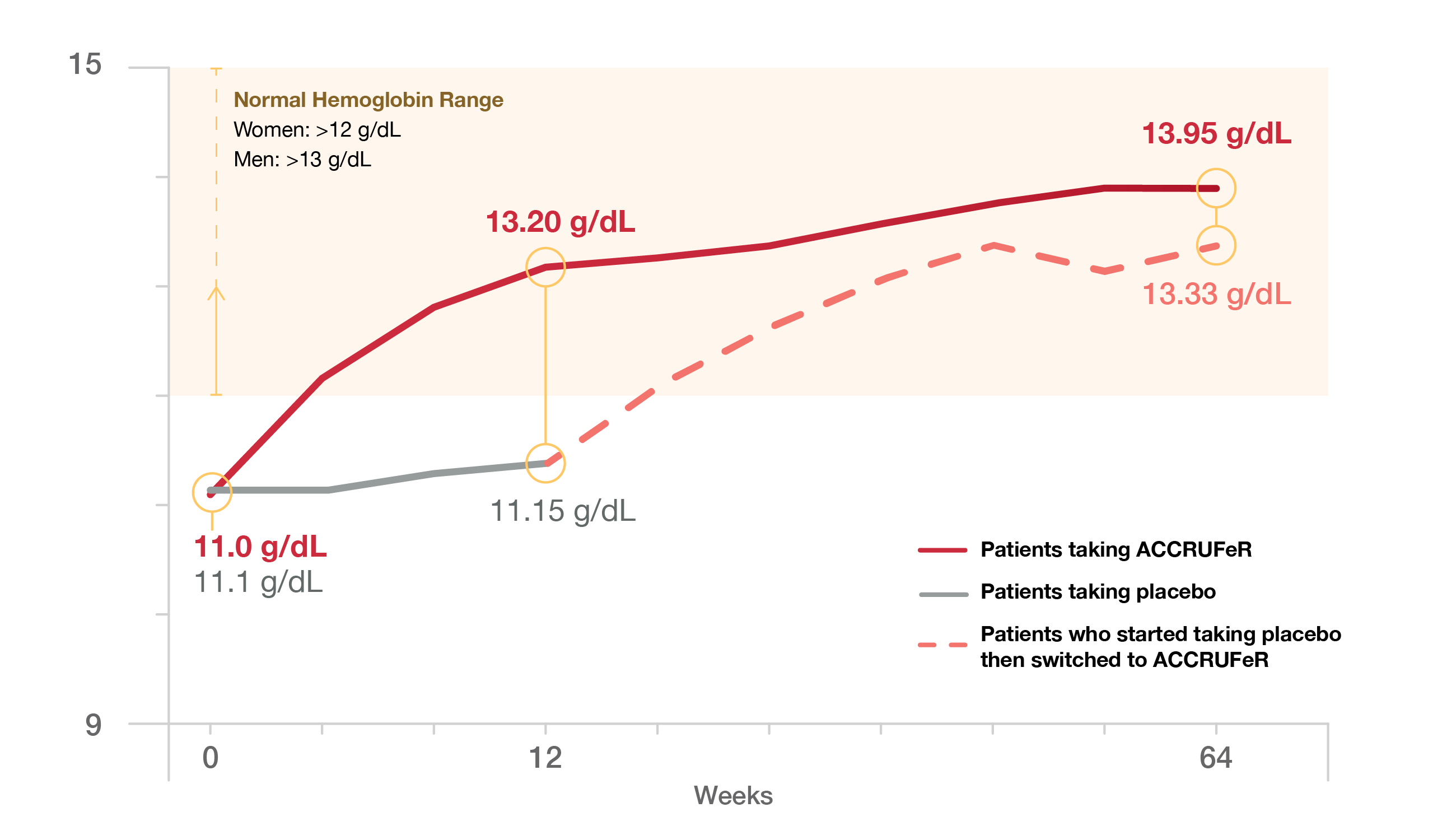
*At Week 12, patients on placebo switched to ACCRUFeR, and patients already on ACCRUFeR continued for an additional 52 weeks.
The study started with 64 patients taking ACCRUFeR and 64 taking the placebo. By the end of the study, 71 patients were still taking ACCRUFeR (35 from the original ACCRUFeR group and 36 from the group that switched from placebo to ACCRUFeR).
Studies of patients with iron deficiency anemia and CKD
After 16 weeks
Patients taking ACCRUFeR saw an increase in their hemoglobin levels, iron stores (ferritin), and available iron (TSAT).
ACCRUFeR increased hemoglobin* by
compared with a 0.02 g/dL decrease for patients on a placebo.
*Average change at Week 16
Hemoglobin Levels Over Time*
Patients who switched to ACCRUFeR from the placebo at Week 16 also had improved hemoglobin levels.
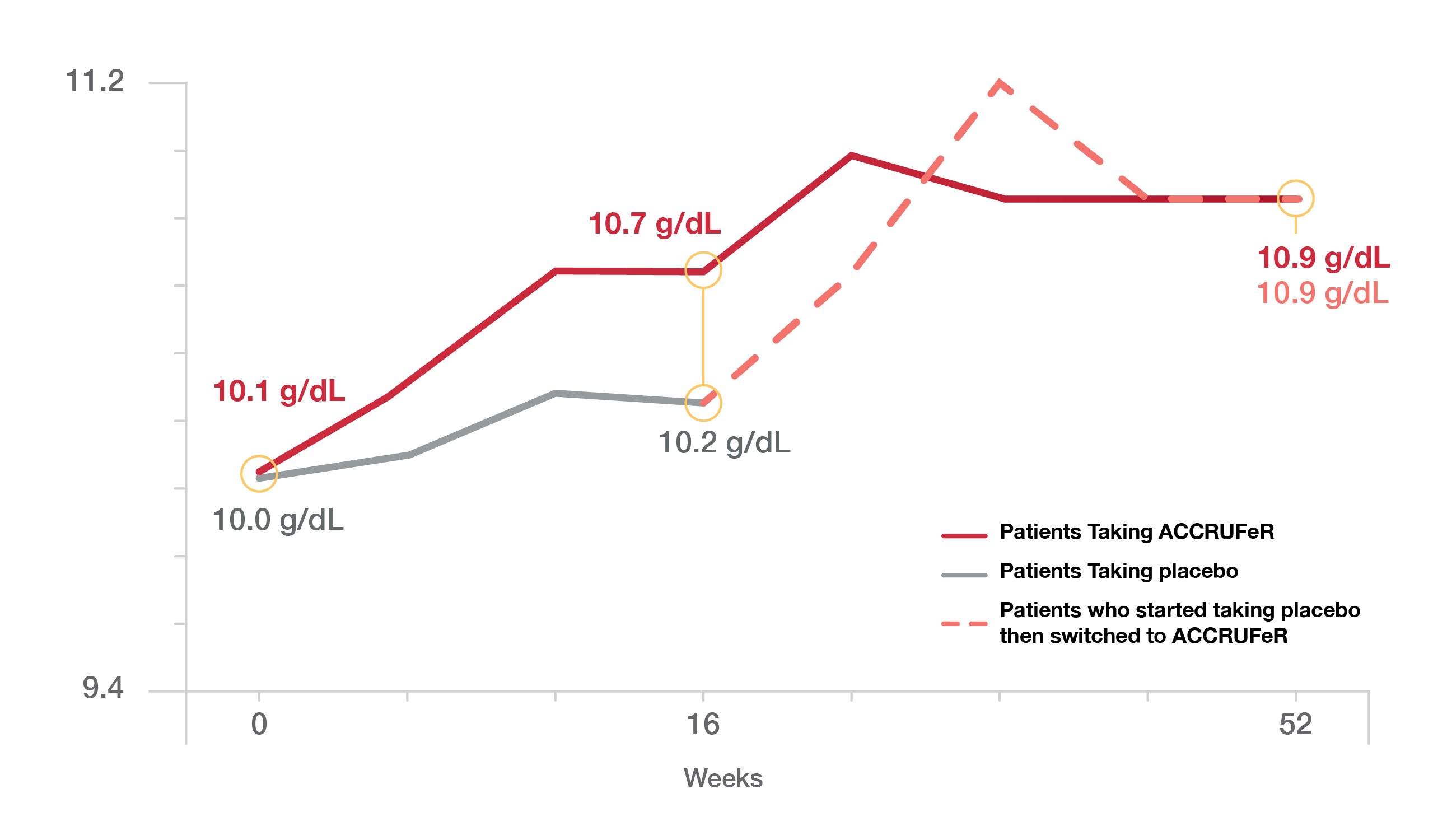
*The study started with 111 patients taking ACCRUFeR and 56 taking a placebo. By the end of the study, 97 patients were still taking ACCRUFeR (67 from the original ACCRUFeR group and 30 from the group that switched from placebo to ACCRUFeR).
Study Descriptions
- The studies included 128 patients with iron deficiency anemia — 70 who also had Crohn’s disease and 58 who also had ulcerative colitis.
- Patients in the studies had previously stopped using other oral iron products because of their lack of effectiveness or an inability to tolerate these products.
- The studies lasted for 12 weeks initially and were extended another 52 weeks.
- The main goal of the studies was to measure the average change in patients’ hemoglobin levels from the start of the trial to week 12.
- The studies also measured average change in ferritin levels and TSAT and normalization of hemoglobin concentration.
- The study included 167 patients with stage 3 or 4 chronic kidney disease who were not on dialysis and iron deficiency anemia.
- The study lasted for 16 weeks initially and was extended another 36 weeks.
- The main goal of the study was to measure the average change in patients’ hemoglobin levels from the start of the trial to week 16.
- The study also measured average change in ferritin levels, TSAT, and serum iron levels.
Tolerability and side effects
Results made possible by treatment you can tolerate
Up to 70% of patients have reported quitting traditional oral iron supplements due to gastrointestinal reactions. If this is you, you're not alone.
ACCRUFeR’s “maltol shield” helps substantially minimize side effects. However, some patients experienced mild to moderate side effects, including gas, discolored stool, stomach area discomfort or bloating, stomach pain, nausea, diarrhea, and constipation. Each of these side effects occurred in fewer than 5% of patients taking ACCRUFeR.
Fewer than 5% of patients stopped taking ACCRUFeR because of side effects.
